This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Starting a Healthcare Electronics Development Project: Key Points You Need to Know


Andrey Solovev
Chief Technology Officer, PhD in Physics and Mathematics

Nina Bibikova
IT Writer, Specializes in Electronics and Software Development Topics
Life expectancy increases worldwide, and the healthcare device market grows with it. Global Market Insights says the global medical electronics market was valued at $154.5 billion in 2022. It is projected to grow at an annual CAGR of 6.5% to around $298 billion by 2032.
So, medical electronics design and device application development are constantly in high demand. The very concept of a medical electronic device includes a bunch of devices that vary in cost, complexity, and purpose—from a wearable tracker to the most complex medical equipment.
All this gives plenty of scope for medical electronic device development based on individual requirements; the main thing is to know where to start and what to pay attention to.
Today, we are going to analyze medical product development requirements and features.
Healthcare Electronics Specifics
Let's just start by finding out how healthcare electronic devices differ from regular electronics.
Undoubtedly, the first thing that makes medical devices different from all other electronics is their increased reliability and safety. Mechanisms designed to support and improve the patient’s health must be tough and durable. They must work flawlessly and never cause harm to people.
Medical electronics need to process health data in real time as quickly as possible. Precious seconds are needed to provide emergency care and can save the patient's life.
Integra Sources, a company that develops custom electronics and software for various purposes, including healthcare, knows how to make real-time data processing electronic devices. We will help you turn your idea of a high-performance healthcare device, system, or app into reality.
Our healthcare projects include creating a bracelet with an alert button. The ultra-low-power, BLE-enabled wearable device allows patients to call for help by pressing an emergency button. The bracelet transmits an alarm signal via Wi-Fi or Bluetooth to a nearby beacon that sends it to the hospital server.
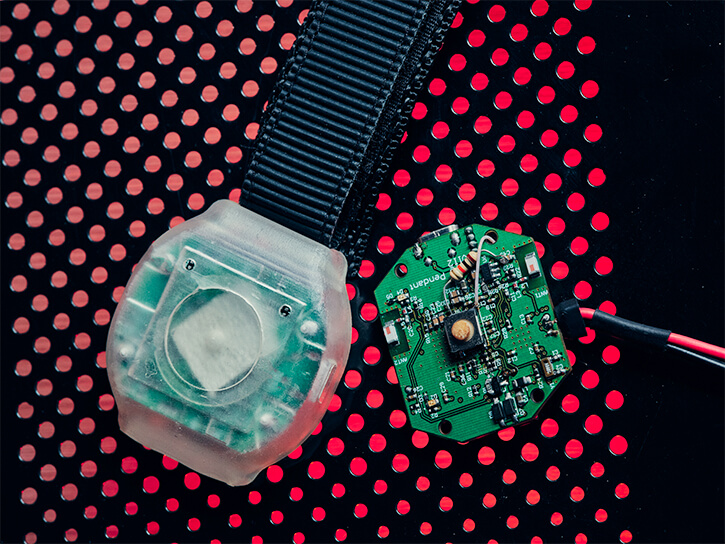
The server calculates the patient's location and passes on a message to the staff. Life-saving assistance can be provided immediately since the minimum possible time passes from pressing the button to receiving the signal by medical personnel.
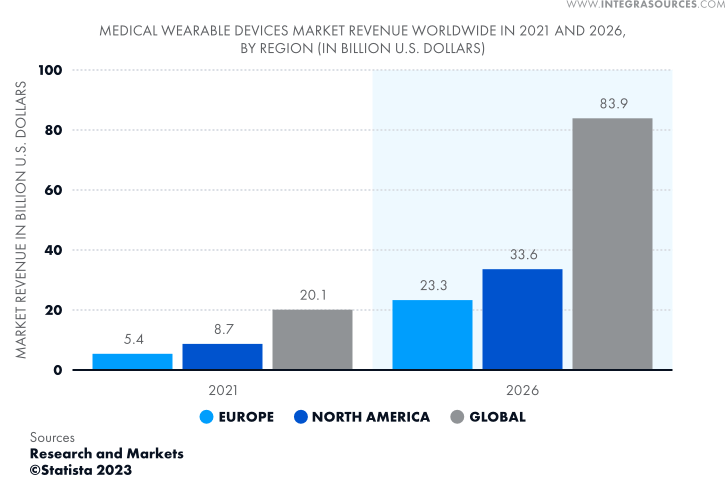
The global market for medical wearables is growing impressively. It is expected to quadruple in 5 years from 2021 and reach $83.9 billion by 2026, as Statista predicts. The North American market is in the lead, accounting for around 40% of global wearable medical device revenue.
Various network technology support is a feature that is closely related to high performance. For example, if the Wi-Fi connection is lost, the device instantly switches to a mobile network without sacrificing functionality, performance, or control.
Data clarity is also not a whim but a must-have condition. Be it a mobile app screen or an HMI healthcare device display, information must be presented in a user-friendly manner. Neither medical staff nor patients can quickly assess the information, getting lost in confusing menu choices.
Other frequently needed medical electronics features may include:
- low power consumption;
- sterilization and disinfectant resistance to withstand frequent cleaning;
- lightweight;
- compact size;
- mobility;
- portability;
- mechanical damage resistance;
- along-lasting appearance.
Medical E-Device Types
All electronic devices for healthcare can be divided into four large groups according to the purpose of use:
- therapeutic;
- diagnostic;
- patient monitoring;
- others.
The therapeutic segment accounts for almost half of the medical electronics market due to the aging population and the growing need for chronic disease care.
The rise of AI-based medical electronics, including those that patients can use at home, drives further therapeutic branch growth.
We applied computer vision algorithms to develop an intelligent system for detecting skin cancer with a smartphone. The system uses an iOS app and a special smartphone camera lens to diagnose skin cancer. Stunning diagnostic accuracy of 80% and processing time of less than 0.1 seconds are made possible by OpenCV-based algorithms developed in C++, wrapped in Objective-C, and integrated into the iOS mobile app.

In addition to grouping by purpose of use, all medical electronic devices are divided into classes of potential danger to consumers. The US Food and Drug Administration (FDA) divides medical electronics into four classes based on the potential risk to the patient and the degree of control required to ensure the safety and effectiveness of the various types of devices.
47% of medical devices fall under the class I category with the lowest possible risk to the patient. 95% of class I devices are exempt from the regulatory process.
43% of medical devices fall into the class IIa or class IIb categories of medium health risk. Most class II products must go through a premarket notification process, aka 510(k).
Class III covers high-risk medical devices, most of which must undergo premarket approval (PMA). These are cochlear implants, pacemakers, defibrillators, and other precision equipment.
The design and development of class II and class III medical devices is accompanied by more stringent requirements for component selection and electronics certification.
Certification
Companies that provide medical device design and development services rely on International Electrotechnical Commission (IEC) standards, namely IEC 60601-1 and IEC 60601-1-2 when designing electronic products for healthcare. IEC62326-1:2002 specifies capability approval (CA) procedures for printed boards. Only a device that meets these standards can be certified.

Companies that design and manufacture healthcare electronics must meet the strict requirements of the International Organization for Standardization (ISO).
ISO standards for medical electronic devices include the following:
- ISO 9001 sets out quality management system (QMS) requirements;
- ISO 13485 specifies quality management system requirements for medical devices;
- ISO 14971 specifies the risk management process in the medical device industry;
- ISO 62304 sets out the life cycle requirements for medical device software;
- ISO 10993 is used to assess the biocompatibility of medical devices and materials.
- ISO 20417 defines requirements for the identification, labeling, packaging, and accompanying information of medical devices.

Consumer electronic devices for healthcare must have so-called electrical certifications: FFC, CE, UL, RoHS, or REACH. You can learn more about consumer electronics certification in the US and EU in our previous post.

Embedded healthcare software may also be subject to additional certification. For example, one of our projects, the device for monitoring after-surgery tissue repair, required C software to conform to the MISRA-C:2012 coding standard to ensure embedded system code safety, security, portability, and reliability.
All electronic healthcare devices – simple digital thermometers, fitness bracelets, personal alarms, complex implantable systems, and diagnostic equipment – must be safe for human life and health. That is why medical hardware design and software development require getting through numerous reliability and operational safety tests (EMI/EMC, ESD, power supply immunity tests, etc.), studies, and mandatory certification.
Before Starting a Project
Technically, the process of medical electronic device creation is not much different from electronics design for other industries.
A company or individual who comes up with an idea to create an embedded system, an IoT solution, a mobile app, or system software for healthcare should test their business concept for vitality, study the market and competitors, and choose an outsourcing hardware design and software development company (or prototype the solution in-house).
However, each step leading up to a medical device prototype development project has its own unique industry-based characteristics.
First, you can't just come to the developer with an idea of a medical electronic product. No matter how hard engineers try to understand the intricacies of medical issues, they will never come close to the necessary medical expertise. You should have your own in-depth knowledge of medicine or qualified medical consulting assistance.
Then, you should find out all the matters related to certification, which depend not only on the device's purpose and operating environment but also on the country of origin. These issues directly affect product creation and component selection processes.
Here is an example from one of our projects. Our US client wanted to use US-certified AC LED lamps in our healthcare IoT project. The IoT system is designed to operate in high humidity. So, we needed to pick up a transformer that met several requirements. It had to be suitable for driving AC LED lamps and be approved for use in a damp environment.
We met all the requirements and installed a humidity-resistant FS12-1600-S2 transformer that reduces the voltage to 12 V.
If you are planning to develop an embedded system, then you need to choose an operating system for it. Open-source or proprietary, the choice is up to you. To learn more about Linux OS for medical devices, read our blog article.
From all of the above, it can be seen that the client should come to the outsourcing development company with a ready-made project requirements specification for a healthcare solution. It's one of the rare cases when technical specialists can only make adjustments to an existing requirements document instead of creating one from scratch.
Risks and challenges
Any hardware and software creation process has its own risks. Providing healthcare electronics design and software development services is no exception. Next, we list the most common medical project threats:
- Certification issues. Your healthcare system, device, or embedded equipment may not get certified. There can be many reasons for that: wrong component selection, design errors, developers' lack of certificates, and many more. The key to avoiding such threats is outsourcing specialists' skills and experience, as well as a thorough-prepared project spec.
- A healthcare electronic device is not safe for patients. This is a genuinely terrible case, but discovering defects during prototyping is a much better scenario than during or after mass production. The only way out is to conduct thorough testing at all project stages.
- Your healthcare electronic solution has had no market success. Success is made up of many elements: luck, fortunate circumstances, intuition, and, of course, careful preparatory work. So, you'd better not ignore the IT project discovery phase, as it will help you correctly assess project viability and market potential.
- The system requirements are incorrectly defined. Vanity and constant rush, as well as a lack of skills and experience, can cause errors in specifying software features, functions, and system requirements. As a result, the developed software may not meet the software environment or hardware requirements. You can reduce such risks to zero by carefully carrying out preparatory work and choosing a qualified development team.
- Software errors. The software may fail during operation, which can lead to serious problems for medical staff and threaten the patient's health.
The development team should pay particular attention to the security and stability of software for medical devices, embedded systems, and IoT solutions. The right choice of programming language and tools, coupled with frequent code testing, will help create software that will work properly for years.
In most cases, we choose C/C++ because of its flexibility, high speed, and scalability, which allows us to create high-performance and reliable solutions.
Let's take a look at the challenges faced by medical electronics and software developers (ourselves included).
- Integrate software into an existing system
Medical software often needs to be included in the health service facilities' existing software system. In such cases, the development team applies software languages and tools and creates interfaces, menus, and navigation so that the new software gets integrated into the overall hierarchy.
- Ensure data transfer speed and continuity
Any electronic device, sensor, or software for medicine must operate quickly and stably under all conditions.
When developing firmware and an Android app for a wearable ECG device, our engineers had to ensure real-time data transfer from the device to the app via Bluetooth.

We needed to ensure continuous data transfer even if there was a temporary loss of connection to the app. That was a challenge. To save data, we implemented temporary data storage in flash memory.
We also encountered a problem related to the Bluetooth channels' data transfer speed. Our team implemented compression provided by the encoder and decoder to achieve the required speed.
- Ensure the device has low power consumption
Customers often ask us to make the device work on a single battery charge for as long as possible. This requirement is not always easy to meet.
Above, we already described the bracelet with an alert button we developed. One of the customer’s requirements was that the bracelet operate for two weeks without recharging on a 360 mAh battery.
We achieved the device's low power consumption by choosing a microprocessor without excessive functions and selecting its energy-saving operating mode without background activity.
- Fit everything needed into a tiny case
The trend toward device miniaturization in healthcare electronics development services is gaining momentum. It takes incredible engineering skills to put everything on the PCB and meet strict size requirements.
Medical Hardware & Software Development Features
The development of hardware and software in the field of medicine has its unique characteristics, but it can still be fitted into the general IT product development framework.
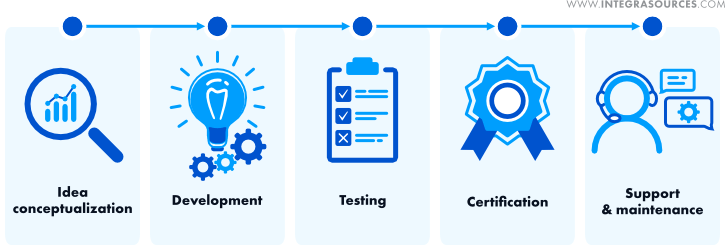
Target market research and idea conceptualization
At this stage, you determine the target audience, market requirements, and trends and formulate your product idea. Medical electronic devices and software applications should be easy to use.
In the case of medical electronics design, you should also determine physical and operational parameters: device size, case material, power supply, safety, service life, etc.
Consider the certification requirements. This step is especially vital for hardware design. It is necessary to take into account all the regulatory authorities' requirements.
Medical product development
The hardware development stage usually takes longer and has more iterations than medical device software development. Yet, it depends on the project's scale and complexity.
While selecting hardware components, engineers consider not only device operational and functional requirements but also certification ones.
While choosing programming languages, frameworks, libraries, and other software tools, specialists are guided by their skills and expertise, customer requirements, and software compatibility. The software can be linked to the hardware design or be a separate product.
When realizing healthcare software development and medical device IoT development, engineers must take care of data security and minimize the IT product's vulnerabilities to hacking or viruses.
Testing
Strictly speaking, hardware and code in-house tests are carried out continuously throughout the entire development process no matter what kind of project the team is dealing with. But the medical field requires increased attention to hardware and software security, durability, and stability. Electronics must run smoothly since patients' health and lives often depend on them. Health data must be reliably protected from hacking, theft, and damage.
Medicine hardware and software system development almost always requires third-party laboratory testing.
Certification testing lengthens IT solution development and increases the number of iterations, which is one of the key features of the medical electronics development process. And yet, simple healthcare electronic products get certified as consumer electronics, but medical devices and systems must meet many more additional certification requirements.
Support and maintenance
After completing device prototyping and software development, companies provide post-project support for a certain period, eliminating shortcomings and errors. Moving forward, on the software side, the developer can provide updates, system scaling, and adding new features.
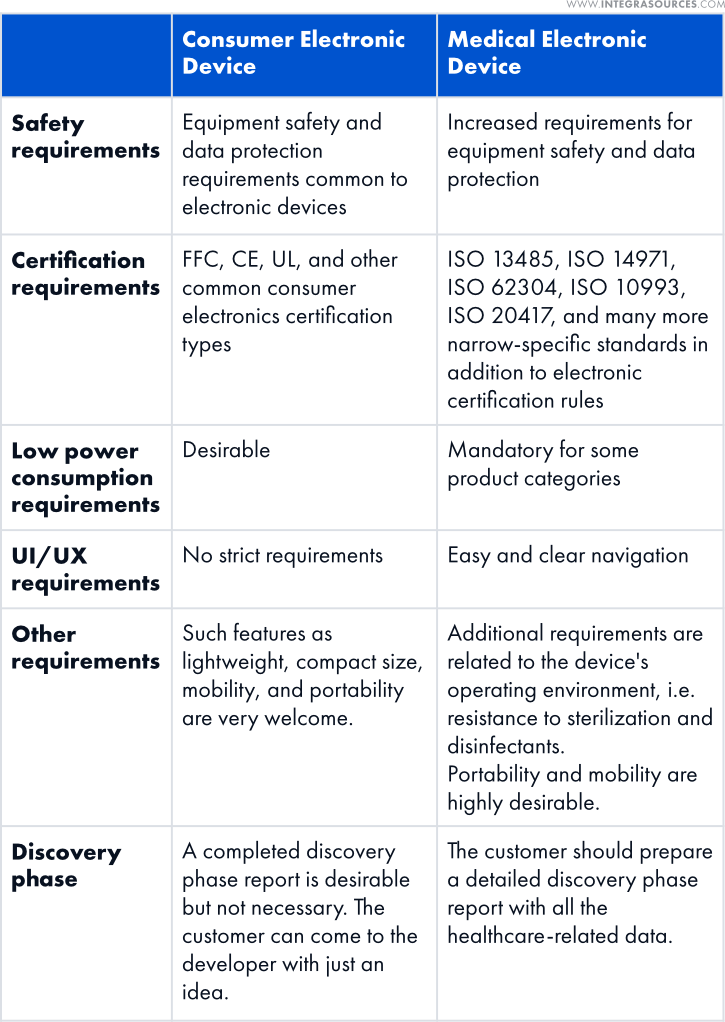
Medical Device Product Development vs. Consumer Electronics Creation
We have picked up the most significant differences between creating consumer electronics and medical electronic devices.
Wrapping up
Building healthcare e-devices and software is an interesting, inspiring, knowledge-enhancing, and promising proceeding. It is similar yet different from all other industry-related development processes.
When you start building a healthcare e-device or app, expect it to take longer and require more resources than creating consumer electronics.
Hardware and software developers must follow both common electronics certifications and numerous stringent medical device certification standards when working on a medical e-solution.
Therefore, you should choose the most highly qualified and experienced outsourcing partners for healthcare product development. Integra Sources' portfolio includes a wide range of healthcare hardware and software development projects. Reach out to us to create a high-performance and safe medical IT solution that benefits human health.
Share this article


Related
materials

5 Common US & EU Consumer Electronics Certifications in Infographics
Certifying a product requires time and money, which is why making a mistake here can lead manufacturers to serious financial...
LEARN MORE 
LEARN MORE 
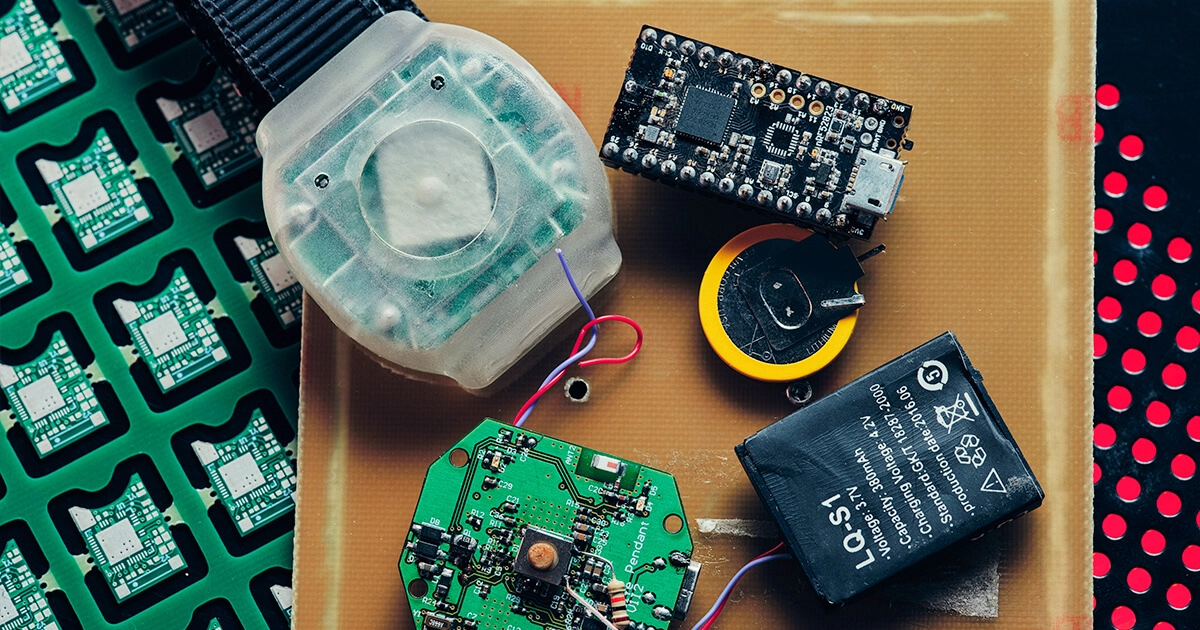
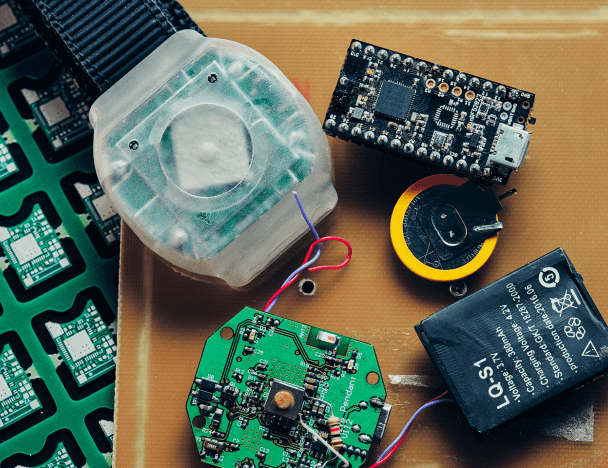
Medical Alert Bracelet That Delivers Assistance at the Touch of a Button
A bracelet designed for patients at military hospitals and clinics in the USA. It notifies hospital staff about an emergency...
LEARN MORE 
LEARN MORE 

Project Development Specification: How To Write the Right One
A successful project starts with writing a competent development specification, which reveals the requirements for the product and the development...
LEARN MORE 
LEARN MORE 




